Design: Figma prototype + appuniversum example
Overview of regulatory statements in GN
Current scope of design:
regulatory statement section in interface
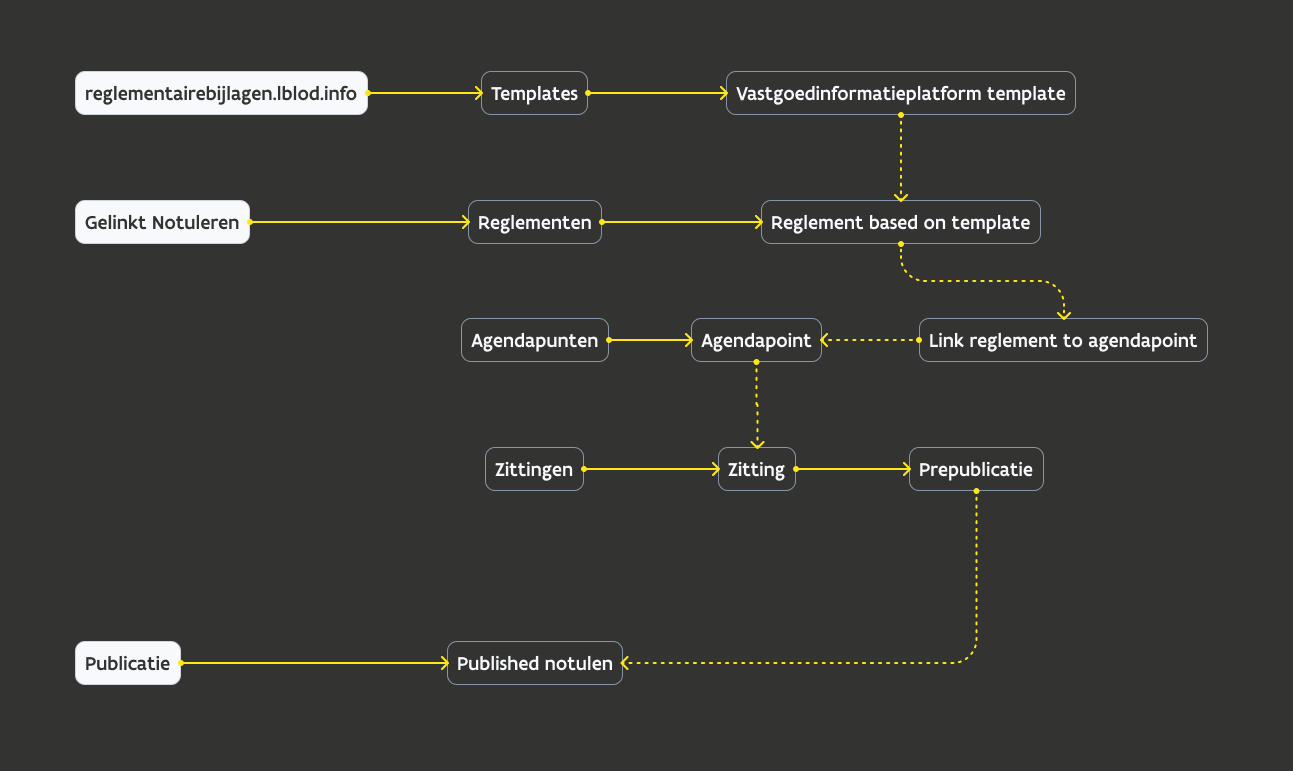
add regulatory statements based on a template
manage regulatory statements: edit, delete, export
link regulatory statements to a decision (agendapoint)
available by a plugin in the editor
in the attachment tab
output in prepublish flow
regulatory statement is inserted as html in the published "notule"/excerpt
Future scope:
versioning
aggregated regulatory statement
General flow
reglementairebijlagen.lblod.info is not included in this design
Prototypes
GN flow in Figma
See also github branch: https://github.com/lblod/frontend-gelinkt-notuleren/tree/prototype/regulatory-statements
RDFA editor structure plugin
Appuniversum component examples
Technical feasability meeting
27/09/22
Participants; Dieter, Judith, Bart, Pieter, Oscar, Niels, Elena
Decisions
No table of contents of the regulatory statement upon prepublication/publication
We remove adding regulatory statements from the attachment tab BECAUSE
We don't know the location where to put it in the decision and through the decision they can still type after it
It's not truly an attachment
We can & should research the current usage of "regelgevende"
We could create it as a plugin & make an embeddable for now.
We do keep the possibility to add an attachment + make sure it's regelgevend
No possibility to create/update a regulatory statement in the decision section
Edit button in the editor; link to the regulatory statement editor
To research
Overview of all regulatory statements; we need to define status --> Need for status "Ready to put on the agenda OR publish" --> status if a draft is finished but not put on the agenda yet. TO USER RESEARCH
Are the attachments currently added through attachments --> Judith to look into when getting a list of attachments
To be updated in current design;
Add design view for publication
Publication side; make sure that an attachment type "regelgevend" is different from a reglement created in Editor
Copywriting change: Concept needs to be "in voorbereiding"
Added on the decision/publication screen: A title in the decision/publication that prefaces there's a regulatory statement following "
Copywriting: Change title from Regulatory statements to Reglementen (until we add other templates than reglementen OR someone disagrees)
Future iterations
Linking to the regulatory statement with a hyperlink
Appropriate name when we add other things than reglementen
Make components drag & drop
Validation if variable fields are filled out
Logic for naming RB for instance "Title" + "City/user created it"
Comments
Can you add a regulatory statement from a new agenda point? Can we add this to the design.
Normally you have a title + template --> should there be a selection for regulatory statement as well? NO, templates are only available in the regulatory statement section, NOT in the decision.
In publications; do we need a table of content?
Is it possible that there's 2 levels of articles; the decisions AND the regulatory statement
YES.
Edit button in the editor; link to the regulatory statement editor
A regulatory statement should always be linked to a decision when added from the attachment tab
Last updated